Introduction: Castleman disease (CD) is a rare lymphoproliferative disorder that encompasses distinct clinicopathological entities. Unicentric form of the disease (UCD) usually involves a single lymph node station, exhibits hyaline vascular (HV) pathological changes with follicular dendritic cell expansion, and may associate with specific and life-threatening complications including paraneoplastic pemphigus (PNP) and follicular dendritic cell sarcoma (FDCS). Plasma cell interfollicular infiltration is absent in the HV subtype as opposed to the Plasma-Cell (PC) or Mixed subtypes. PC subtype has been associated with inflammatory symptoms thought to be secondary to plasma cell infiltration and represents the majority of HHV8-negative “idiopathic” multicentric forms of the disease. Interestingly, patients with PC-UCD behave as PC-MCD, suggesting a stronger role of histology rather than Unicentric vs Multicentric phenotype in disease expression. We here studied the phenotype of HV-MCD, a rare subtype of CD characterized by hyaline-vascular pathological changes involving 2 or more lymph node stations.
Methods: Patients were screened through the French national reference center for CD between January 1994 and June 2023. Cases were followed from first symptoms to last follow-up visit. Comparators consisted of patients with HV-UCD and patients with PC/Mixed iMCD (P/M-iMCD). All cases and biopsies were reviewed by three expert clinicians and one expert pathologist.
Results: Sixteen patients with a diagnosis of HHV8-negative HV-MCD were identified. All patients had supra- and infradiaphragmatic involvement. Four were excluded because of a subsequent diagnosis of POEMS, systemic lupus erythematosus, tuberculosis, or inborn error of immunity. The 12 remaining patients were considered as having HV-iMCD and compared to 101 patients with P/M-iMCD and 139 patients with HV-UCD. Characteristics and comparison between the 3 groups are depicted in Table 1.
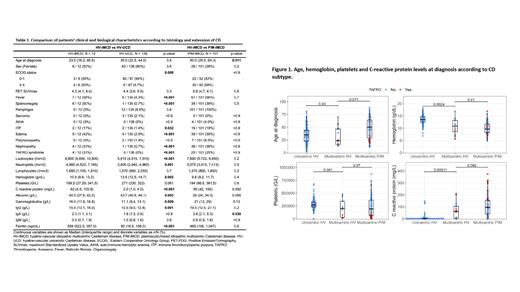
When compared to HV-UCD, HV-iMCD displayed frequent systemic involvement with significantly higher ECOG, higher frequency of fever and splenomegaly, and higher levels of C-reactive protein, gammaglobulins, leukocytes and ferritinemia ( Figure 1). Age at diagnosis was not different between HV-UCD and HV-iMCD. No cases of TAFRO syndrome were observed in HV-UCD whereas 4 cases were observed in HV-iMCD. All cases of PNP (n=12) and FDCS (n=3) were observed in the HV-UCD group.
We observed a younger age at diagnosis in HV-iMCD compared to P/M-iMCD (median 24 [range 13-70] vs median 50 [range 11-88] years respectively, p = 0.01). We also noted a trend towards lower levels of C reactive protein and gammaglobulins as well a higher albumin level in HV-iMCD vs P/M-iMCD but these differences were not significant ( Figure 1 and Table 1).
Treatments were similar between HV-iMCD and P/M-iMCD with prescription of steroids or anti-IL6R as first line therapy in 8/12 (67%) and 61/101 (60%) respectively. As expected, surgery was the first line therapy in 98/139 (72%) of HV-UCD patients. One patient with HV-iMCD and two with HV-UCD died (logrank p = 0.10), whereas 13 died in the P/M-iMCD group (logrank p = 0.8).
Conclusions: These data altogether indicate that HV-iMCD appears to be a distinct entity in CD landscape, sharing pathological aspects of HV-UCD and clinical/biological features of P/M iMCD. In line with this view, there seems to be no overlap between HV-UCD and HV-iMCD-related complications. The findings also suggest that nodal PC infiltration is not sufficient to explain the inflammatory pattern of iMCD.
Disclosures
Meignin:EUSAPHARMA: Consultancy. Terriou:Alexion: Honoraria; Sobi: Honoraria; Eusapharma: Consultancy. Viallard:EUSAPHARMA: Consultancy. Galicier:EUSAPHARMA: Consultancy; AMGEN: Consultancy. Oksenhendler:EusaPharma: Consultancy; CSL Behring: Consultancy.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal